
CEO Oerlikon AM Europe GmbH (a company of Oerlikon Group)
AMPOWER supports you on your way to operational excellence in Additive Manufacturing by setting up a qualified production including upstream and downstream process steps. Our approach complies to the specific standards and management practices in your industry as well as best-practices and general guidelines for Additive Manufacturing. We guide you through setting up a quality management system and assist in preparation of the necessary documentation. Through our experience you will significantly accelerate your qualification process and successfully pass your audit.

The qualification of Additive Manufacturing poses a major challenge to many companies. The complex norm landscape, limited experience and lack of best practices lead to various uncertainties. Ampower pursues a clearly structured consulting approach to support its customers on the way from a first gap analysis until successfully passing the audit.
Evaluation of as-is status of quality management and comparison with your industry and customer requirements
Setting up or further develop an AM specific quality management according to all requirements
Supporting machine acceptance and qualification at your own production site or auditing of external suppliers
Our gap analysis is offered at a fixed price and gets you started on the way to a qualified Additive Manufacturing production. The gap analysis highlights all deviations from your set-up to your industry and customer requirements. Based on these results, we prepare an action plan for the production and quality management systems with the goal of successful auditing according to your customer and industry requirements. In a follow-up project we can support you in the mitigation of identified deviations and guide you on your road to achieve operational excellence.
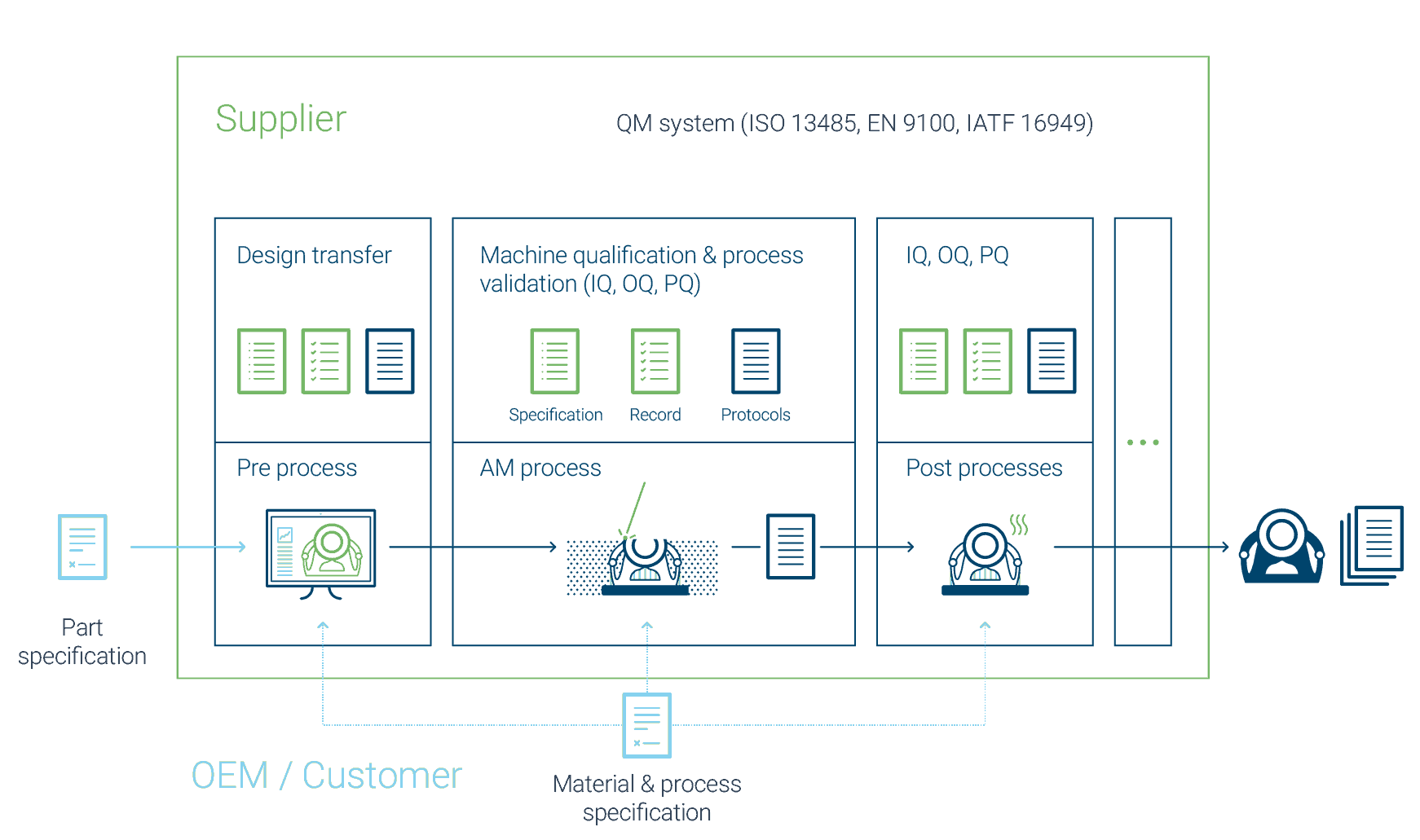
The part specification defines all properties of the component. The user specifies these, for example in the form of a technical drawing.
The material specification defines the properties of the input material as well as the material resulting from the process. These can be strength values as well as chemical composition and density. The Process specification defines parameters and requirements of the manufacturing process.
Each delivered component or batch contains a documentation that proves that the components meet the specifications.
For the external production of additive components, specifications defining all requirements regarding the component, the material and its manufacturing process should be created. This applies in particular to regulated markets such as medical or aviation, but also to general engineering.
We take care of the development of component specifications as well as process and material specifications. This means that we support you in the creation of drawings, component datasets and internal specifications for materials and manufacturing processes. In addition, we can manage the selection and qualification of the complete supply chain for you.
If you supply additive components to regulated or demanding markets, you need to qualify your manufacturing processes. We analyze your customer specifications and transfer them into actions for your quality management for Additive Manufacturing.
Our consulting results in a complete set of necessary business and production processes including their documentation. For example, the preparation of a risk analysis, safety and health assessment, the development of work instructions and production protocols. In addition, we train your employees to establish the right mindset and culture for a successful Additive Manufacturing production.
We qualify according to your specific industry and customer requirements. Common examples for standard practices of qualification are found in medical, aviation and automotive industries.
General quality management in medical technology is defined by ISO 13485. The specifications for the quality management of Additive Manufacturing are based on the component specifications and international guidelines such as those of the U.S. FDA.
The EN/AS 9100 provides the framework for quality management in aviation. In addition, the OEM specifies requirements for the qualification of the Additive Manufacturing process.
The IATF 16949 defines the general framework of quality management in the automotive industry. To date, only few specifications have been defined by automotive OEMs to further define requirements for Additive Manufacturing.